CARDIOVASCULAR DISEASE
Restoring mitochondrial function for cardioprotection
Role of Mitophagy in Heart Failure
Mitophagy, the selective degradation of damaged or dysfunctional mitochondria via the autophagy pathway, plays a crucial role in maintaining cardiac health and protecting the heart from stress-induced injury. In cardiomyocytes, which have high energy demands and limited regenerative capacity, efficient removal of defective mitochondria is vital to preserve mitochondrial quality and prevent the accumulation of reactive oxygen species (ROS), which can lead to cell death.
European Heart Journal., 2025 Jan 21;46(4):380-393. doi: 10.1093/eurheartj/ehae782 PMID: 39601359
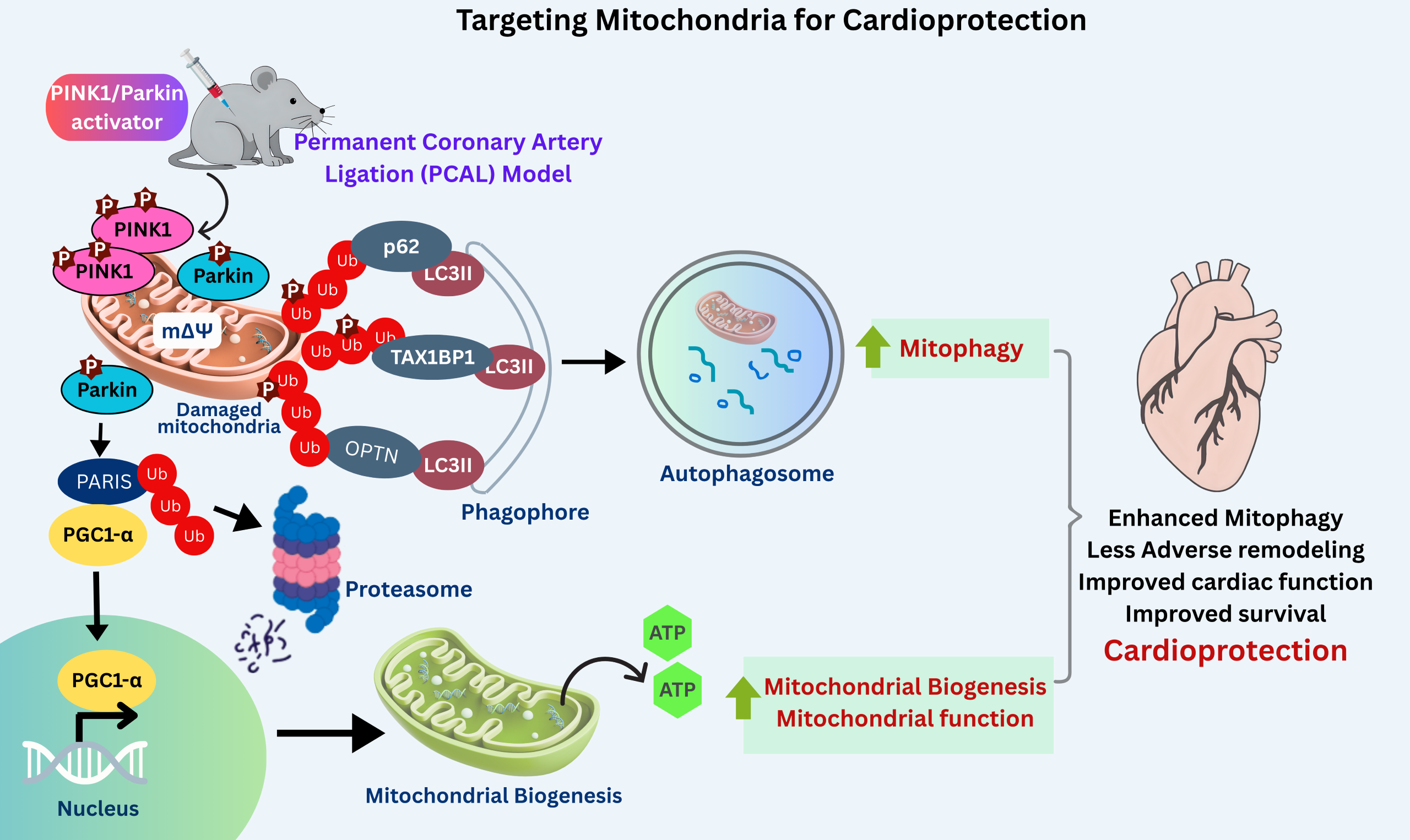
Figure 7. PINK1/Parkin activators are cardioprotective. In a mouse model of heart failure, treatment of mice with PINK1/Parkin activator promotes ubiquitination of OMM proteins which are recognized by mitophagy adapter proteins such as p62/TAX1BP1/OPTN resulting in recruitment of LC3II containing phagophores and subsequent formation of autophagosome and degradation of damaged mitochondria are degraded by mitophagy. Activated Parkin also ubiquitinates and degrades PARIS a repressor of PGC1-α, the master mitochondria transcription factor. PGC1-α enters nucleus and drives transcription of genes resulting in mitochondrial biogenesis. PINK1/Parkin activators improve cardiac function, survival and reduce adverse remodeling ultimately resulting in cardioprotection.
A Pathway Toward Cardioprotection
During cardiac stress conditions such as ischemia-reperfusion injury, enhanced mitophagy helps to limit mitochondrial dysfunction and reduce inflammation, thereby contributing to cardioprotection. Key regulators of mitophagy, including PINK1 and Parkin, coordinate the tagging and clearance of compromised mitochondria. Dysregulation of this pathway has been implicated in the progression of heart failure, making mitophagy an important therapeutic target for improving cardiac resilience and function.
